Improve Pharmaceutical Efficiency and Transparency with Daml Driven Solutions for Supply Chains and Patient Data
Chander Damodaran from Brillio discusses how Daml smart contracts can accelerate the collaboration and automation of multiparty business processes in clinical trials and pharma supply chains
As 2021 begins to take flight, the entire world has turned their attention to Moderna, Pfizer-BioNTech, and many other pharmaceutical organizations to slow the spread of COVID-19 and its variants. The speed with which the vaccine was researched, developed, and trialed shows the paramount effort and collaboration required among external entities to create and mass distribute a vaccine.
While this is a unique time for pharmaceutical companies, it also sheds light on the continued need for digital transformation within healthcare to improve patient care. From audits to supply chain logistics, the entire pharmaceutical industry runs on disparate platforms that create data islands, increasing errors across research data and complicating the track and trace procedures of drugs. Now, more than ever, pharma teams need a way to access patient data for clinical trials, efficiently track trial consent, distribute trial data with external parties, enact drug recalls quickly, and ensure quality along the supply chain all while maintaining compliance with an ever growing list of regulations. This can only be done by enabling communication and data sharing between the multiple parties involved.
Digitally transact in a secure and permissioned environment
Smart contracts offer a path for more automated and secure multiparty business transactions, and provide a means for pharmaceutical entities to view and share data with parties based on permissions defined in the code. Brillio’s innovation team uses Daml smart contracts across multi-party optimization projects for several reasons:
- Daml smart contracts are unique in their ability to abstract away the underlying infrastructure across databases and distributed ledgers, enabling pharmaceutical companies to leverage the systems they currently have and build multiparty applications that easily port to blockchain (if the company moves in that direction).
- Daml interoperates across blockchains and databases, enabling integrations with multiple infrastructure providers and communication between segregated domains. This creates more connected and seamless clinical research and supply chain operations.
- Daml was built from the ground up to support distributed logic, so the code automatically handles distributed concerns while developers only have to code business logic. This means rights, obligations, and authorization are inherent in the code and ensures the right information is shared with the right party at the right time.
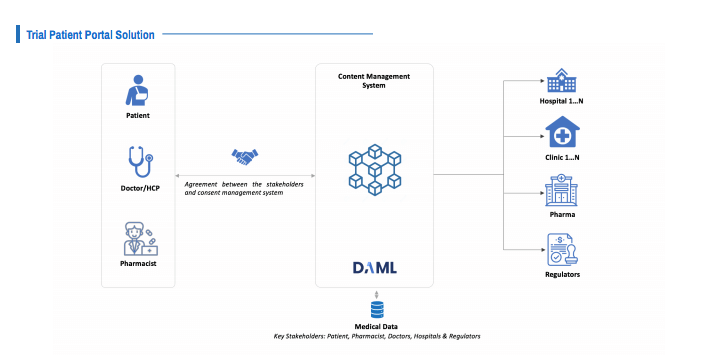
A trial patient portal for improved clinical research processes and data sharing
In the recent “Innovative Healthcare Systems for Clinical Trials and Drug Delivery” e-book, Brillio and Digital Asset proposed multiple Daml-driven solutions, including an end-to-end trial patient portal for clinical research teams. This solution uses the Daml smart contract development framework to build a distributed application with the following features:
- A secure patient portal for collecting and storing patient records (necessary for future audits and data sharing with healthcare providers). Through Daml’s privacy-first framework, clinical trial participants can log their drug reactions, digitally engage with the research team, and receive notifications in a trusted environment. Daml enables the research team to share information with healthcare providers on a need-to-know basis, hiding information they are not permissioned to receive via Daml’s framework.
- A patient consent model where patients accept the terms of the trial in real-time through the application interface. The application stores all patient trial data without the risk of losing paperwork for audits. This ensures the research data is accurate and provides a more transparent log of actions throughout the clinical trial.
- A report configuration interface where research teams can provide documentation and data that adheres to industry regulations.
A supply chain track and trace solution to speed up recalls
Another solution proposed in the “Innovative Healthcare Systems for Clinical Trials and Drug Delivery” is a track and trace application that identifies counterfeit ingredients and enacts speedy drug recalls. This solution would allow manufacturers to notify all stakeholders along the supply chain of contaminated drug batches. It would also cancel all future shipments while notifying patients of the adverse effects prior to consumption. Daml enables the following:
- Track the drug through the entire supply chain from supplier to point of sale, creating a completely transparent end-to-end view of the drug’s lifecycle.
- Facilitate collaboration between all supply chain participants by allowing manufacturers, logistics providers, distributors, hospitals, and pharmacies to transact in real-time with one another, send notifications, and view data based on the rights and obligations outlined in the code for each party.
- Provide direct access to supply chain data for regulators and other industry participants needing to review drug recall notifications.
As we enter an era where technology and healthcare merge, the possibilities are endless. Pharmaceutical companies can leverage cloud technology and smart contracts to support distributed business models that increase the speed of clinical trials, data sharing, and drug delivery. The ability to share data across a shared network without losing the integrity and privacy of that information is only achieved through Daml smart contracts. Brillio’s team of Daml certified engineers can develop custom solutions that not only eliminate manual, expensive processes, but also accelerate innovative care to patients on a global scale.
Learn more about how Brillio and Digital Asset are transforming the pharmaceutical industry in the “Innovative Healthcare Systems for Clinical Trials and Drug Delivery” e-book. Various solutions and workflows are outlined to demonstrate the possibilities for business transformation in pharma.
